One minute to take you to understand the pure water hydrogen generator
Release time:
Mar 24,2022
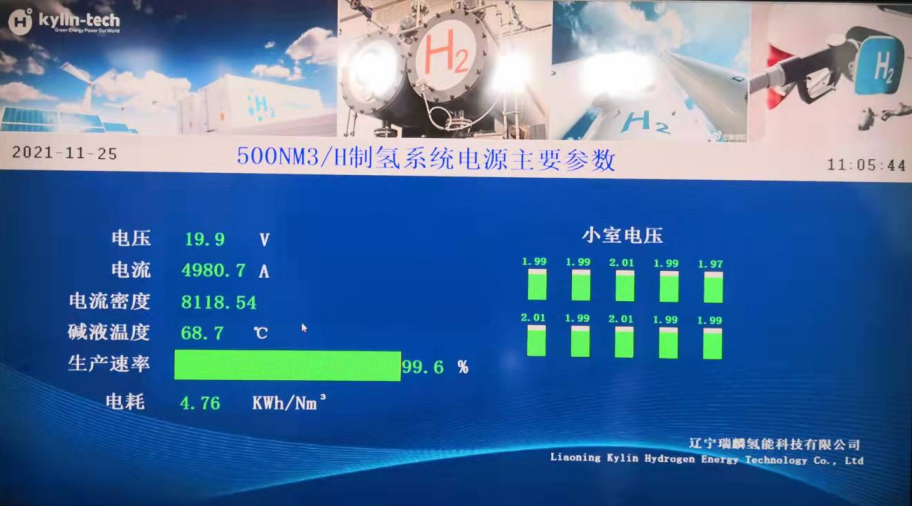
The pure water hydrogen generator consists of components such as an electrolysis cell, a hydrogen/water separator, a collector, a dryer, sensors, a pressure regulating valve, and others. The electrolysis cell is a vessel used to hold the aqueous solution and generate electrolysis reactions. The hydrogen/water separator separates the hydrogen gas generated by electrolysis from the water and removes any entrained moisture through the dryer before it enters the collector for use. The pressure regulating valve controls the reaction rate and level by adjusting the pressure inside the reactor. Hydrogen is flammable and explosive, so special attention should be paid to avoid ignition sources to prevent explosions when using it.
A fuel tank with a defined internal space of a rated volume is equipped with a hydrogen exhaust port connected to the internal space. A catalyst containing hydrogen storage material is stored in the fuel tank, with the catalyst filled in a catalytic reactor. The reactor is equipped with a closed portion that can be used to block contact between the catalyst and the fuel liquid, as well as an open portion in contact with the fuel liquid. Therefore, it can actively adjust whether to generate hydrogen or stop hydrogen generation based on the rise and fall of pressure inside the fuel tank.
Electrolyzed water that meets the requirements (with a resistivity greater than 1MΩ/cm, deionized water or doubly distilled water used in the electronics or analytical industries is acceptable) is fed into the anode chamber of the electrolysis cell. Upon energization, water immediately dissociates at the anode: 2H2O = 4H+ + 2O2-. The negatively charged oxygen ions (O2-) immediately release electrons at the anode, forming oxygen (O2), which is discharged from the anode chamber, carrying some water into the water tank. The water can be recycled, and the oxygen is released into the atmosphere through small holes on the cover of the water tank. The hydrogen protons, in the form of hydrated ions (H+·XH2O), are driven by the electric field force through the SPE ion membrane to reach the cathode, where they absorb electrons to form hydrogen gas. After being discharged from the cathode chamber, the hydrogen enters the gas-water separator, where most of the water carried out from the electrolysis cell is removed. The hydrogen containing trace amounts of water is further dried by a dryer, resulting in a purity of over 99.999%.
Key words:
Related News




